Which Best Describes the Transition From Gas to Liquid
Which term best describes liquid behavior under pressure. Bend the rubber tube to form a concave mirror and place in the ripple tank.
The transition of the solid phase to the gaseous phase without passing the intermediate liquid phase is known as sublimation.

. Gas in a liquid Cliquid in a liquid Dliquid in a solid in an aqueous solution of potassium chloride the solute is. Energy is either removed or added depending on the type of particles. A 108 mL gas sample has a mass of 7796 mg at a pressure of 1140 mmHg and a temperature of 183 C.
Assume ideal gas behavior and a completely insulated system and please donât forget units with your answers. CEnergy is neither added nor removed in this phase transition. A state of matter that consists of loose free moving particles which form the shape set by the boundaries of the container in which the liquid is in.
Energy must be added because particles in liquid move more quickly. The diagram below shows the different phase transitions that occur in matter. This process is called deposition.
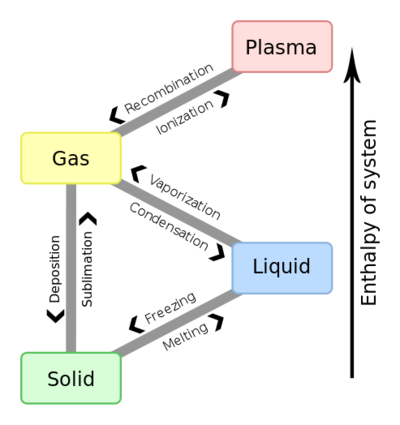
The process by which a liquid changes to a gas b. A phase transition is the transition from one state of matter to another. This happens because the motion of the individual particles.
1gas--solid 2gas--liquid 3liquid--solid 4liquid--gas Chemistry a change in pressure would have the greatest effect on the solubility of a A. Follow these directions and answer the questions. The graph shows the temperature of a pure substance as it is heated at a constant rate in an open vessel at 10 atm pressure.
In which phase transition do molecules move directly from a state involving vibration of particles in a fixed position to a state involving random movement of high. Thus the extra energy need to be released before converting it. Arrow 1 points from liquid to gas.
Sublimation is an endothermic phase transition that occurs at temperatures and pressures below a substances triple point in its phase diagram. They are connected by arrows labeled 1 to 6. Both a and b d.
Water vapor to ice - Water vapor transforms directly into ice without becoming a liquid a process that often occurs on. Of the gas to condense into the liquid. The system is cooled.
3 on a question. There are three states of matter. Which best describes the transition from gas to liquid.
Increasing the pressure on a gas decreases the volume. Gram on the pT plane. 2 on a question.
Set up the ripple tank as in previous investigations. Which best describes the transition from gas to liquid. The process by which a substance changes from the liquid phase to the gaseous phase is known as evaporation.
The process by which a substance changes from the gaseous phase to the liquid phase is known as condensation. The following questions relate to the graph below. Describe the process of vaporization.
Which best describes the transition from gas to liquid. Energy is neither added nor removed in this phase transition. Evaporation is a phase transition from the liquid phase to the gas phase that occurs at temperatures below the boiling point at a given pressure.
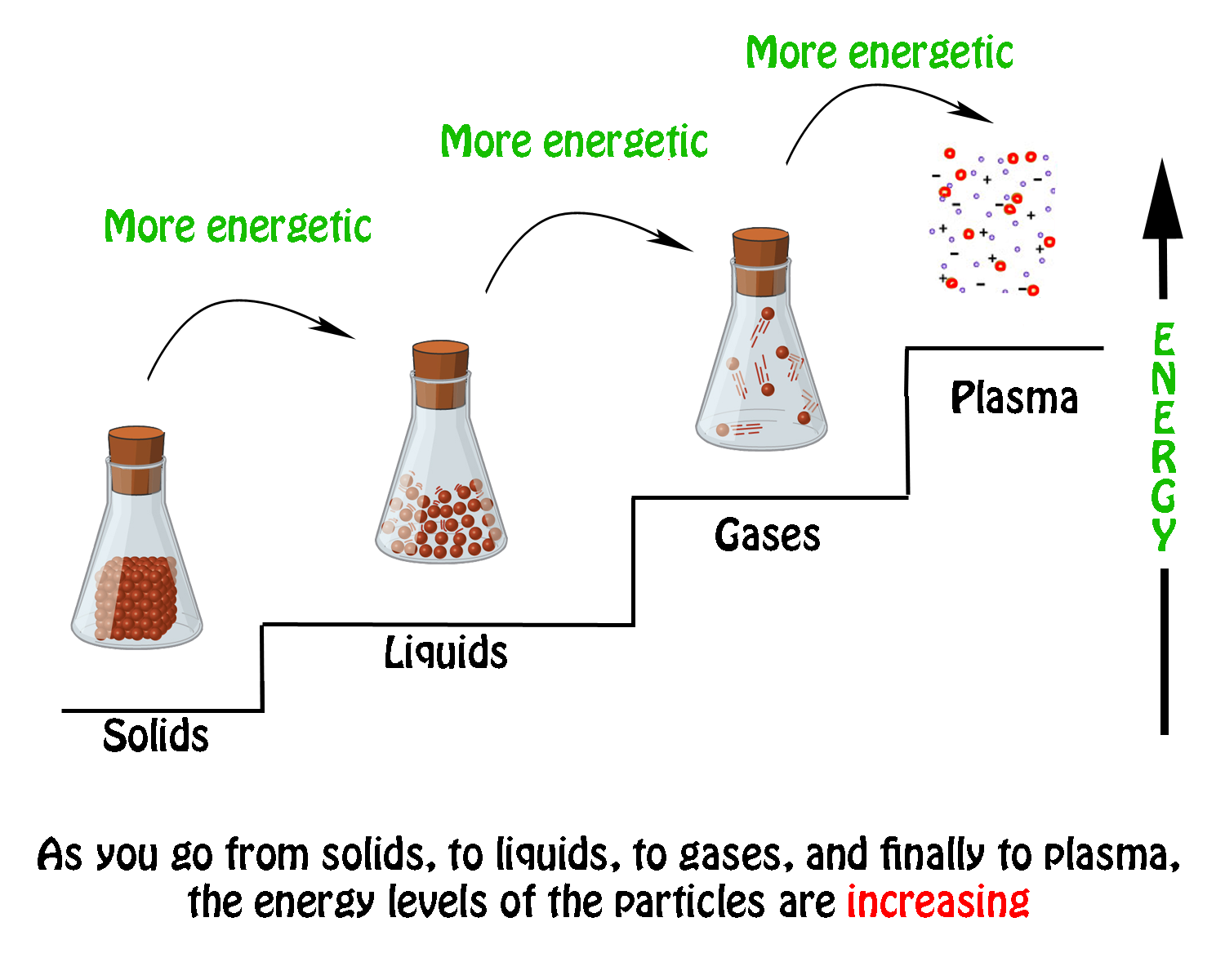
The specific heat of water is 418 JgC. Gas is higher energy state than liq. Liquid solid and gas.
Three bars are shown labeled Solid Liquid and Gas. Energy must be added because particles in liquid move more quickly. Energy must be added because particles in liquid move more quickly.
For molecules of a liquid to evaporate they must be located near the surface be moving in the proper direction and have sufficient. Energy must be removed because particles in liquid move more slowly. Energy must be removed because particles in liquid move more slowly.
Condensation Gas Liquid Sirintra Pumsopa Getty Images. Solid in a liquid B. Which statement best describes the compressibility of a gas.
This photo displays the process of condensation of water vapor into dew drops. Condensation- It is the change of the physical state of matter from gas phase into liquid phase. Energy is neither added nor removed in this phase transition.
Gas molecules inherit large amount of energy in order to move fast as the particles of gas moves faster compared to liquid. Energy must be removed because particles in liquid move more slowly. Commonly the term is used to refer to changes among the basic states of matter.
17 Which best describes the particles of a liquid compared to those of a gas a from SCIENCE 20033400 at Everglades High School. Energy must be added because particles in liquid move more quickly. How much energy was released in joules.
Here the co-existence region is. Describe the process of vaporization. So the transition must remove energy.
Energy is either removed or added depending on the type of particles. 512 The Clausius-Clapeyron Equation We can also choose to plot the liquid-gas phase dia-Tc liquid gas p critical point T Figure 40. Energy must be removed because particles in liquid move more slowly.
The process of transferring from gas to liquid is termed as condensation. For molecules of a liquid to evaporate they must be located near the surface be moving in the proper direction and have sufficient kinetic energy. The process by which a solid changes to a gas c.
Vaporization or evaporation is the process by which molecules undergo a spontaneous transition from a liquid phase to a gas phase. The water level must be below the top of the hose. Energy must be removed because particles in liquid move more slowly.
Energy is neither added nor removed in this phase transition. Arrow 2 from solid to liquid arrow 3 from solid to gas arrow 4 from gas to liquid arrow 5 from liquid to solid and arrow 6. Energy is either removed or added depending on the type of particles.
Solid liquid and gas as well as. Similarly expanding a liquid beyond the co-existence curve results in an meta-stable superheated liquid. In an experiment a scientist burned 100 g of methane and the temperature of the 5000 mL of water in the calorimeter changed from 210C to 266C.
Energy is either removed or added depending on the type of particles. Sublimation- It is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Which best describes the transition from gas to liquid.
500 x 103 J. For the electronic transition from n 3 to n 5 in the hydrogen atom. So ans is a.
Which best describes the transition from gas to liquid. Evaporation is a phase transition from the liquid phase to the gas phase that occurs at temperatures below the boiling point at a given pressure. Condensation the opposite of evaporation is the change in.
It requires the release of energy from the gas molecules to condense them as liquid. Examples of Gas to Solid Deposition Under certain circumstances gas can transform directly into a solid. The substance changes from the solid to the liquid to the gas phase.
In chemistry thermodynamics and many other related fields phase transitions or phase changes are the physical processes of transition between a state of a medium identified by some parameters and another one with different values of the parameters. Key Takeaways Key Points. Which best describes the transition from gas to liquid.
For The Three States Of Matter Solid Liquid And Gas There Are Six Possible Changes Of State Which Changes Of State Are Exothermic And Which Are Endothermic Socratic

0 Response to "Which Best Describes the Transition From Gas to Liquid"
Post a Comment